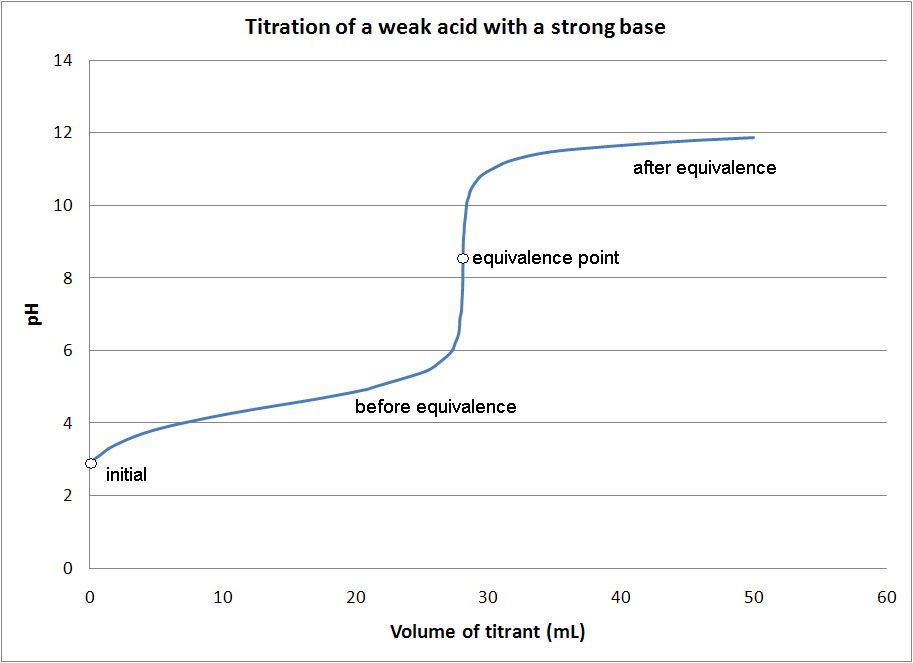
Weak Acid Strong Base Titration Lab Report Discussion . titration of a weak acid with a strong base. Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a. titration of a weak acid with strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. In this particular case, the weak base (colored in green), is. To determine the pka value of a weak acid. titration of a weak acid by a strong base. Conventional setup of a lab titration. titration of a weak monoprotic acid. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh.
from www.writework.com
Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a. titration of a weak acid by a strong base. titration of a weak acid with a strong base. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. To determine the pka value of a weak acid. In this particular case, the weak base (colored in green), is. Conventional setup of a lab titration. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3.
Titration of amino acids WriteWork
Weak Acid Strong Base Titration Lab Report Discussion To determine the pka value of a weak acid. by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. titration of a weak acid with a strong base. To determine the pka value of a weak acid. Conventional setup of a lab titration. In this particular case, the weak base (colored in green), is. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. titration of a weak acid by a strong base. titration of a weak monoprotic acid. titration of a weak acid with strong base. Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a.
From www.vrogue.co
Acid Base Titration Lab Dataclassroom Lab Report Titr vrogue.co Weak Acid Strong Base Titration Lab Report Discussion Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a. titration of a weak monoprotic acid. To determine the pka value of a weak acid. titration of a weak acid with strong base. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m. Weak Acid Strong Base Titration Lab Report Discussion.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Weak Acid Strong Base Titration Lab Report Discussion titration of a weak acid by a strong base. by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. To determine the pka value of a weak acid. the titration of a weak acid with a strong base involves the. Weak Acid Strong Base Titration Lab Report Discussion.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Weak Acid Strong Base Titration Lab Report Discussion In this particular case, the weak base (colored in green), is. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. titration of a weak acid with strong base. titration of a weak acid with a strong base. The titration curve shown in. Weak Acid Strong Base Titration Lab Report Discussion.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Weak Acid Strong Base Titration Lab Report Discussion titration of a weak monoprotic acid. Conventional setup of a lab titration. To determine the pka value of a weak acid. titration of a weak acid by a strong base. by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration.. Weak Acid Strong Base Titration Lab Report Discussion.
From www.poshpooch.ca
Acid base titrations lab report. 24/7 College Homework Help. Weak Acid Strong Base Titration Lab Report Discussion by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. In this particular case, the weak base (colored in green), is. titration of a weak acid by a strong base. titration of a weak monoprotic acid. The titration curve shown. Weak Acid Strong Base Titration Lab Report Discussion.
From www.vrogue.co
Strong Acid Weak Base Titrations vrogue.co Weak Acid Strong Base Titration Lab Report Discussion by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. To determine the pka value of a weak acid. In this particular case, the weak base (colored in green), is. titration of a weak acid by a strong base. Conventional setup. Weak Acid Strong Base Titration Lab Report Discussion.
From www.youtube.com
17.3 Weak Acid Strong Base Titration Curve YouTube Weak Acid Strong Base Titration Lab Report Discussion titration of a weak acid with strong base. Conventional setup of a lab titration. titration of a weak acid by a strong base. by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. In this particular case, the weak base. Weak Acid Strong Base Titration Lab Report Discussion.
From childhealthpolicy.vumc.org
💣 Discussion lab report acid base titration. Lab Report Acid Base Weak Acid Strong Base Titration Lab Report Discussion titration of a weak acid with a strong base. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. by observing the titration of. Weak Acid Strong Base Titration Lab Report Discussion.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Weak Acid Strong Base Titration Lab Report Discussion To determine the pka value of a weak acid. Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a. titration of a weak acid with a strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. In the previous post, we discussed the. Weak Acid Strong Base Titration Lab Report Discussion.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Weak Acid Strong Base Titration Lab Report Discussion titration of a weak acid with a strong base. titration of a weak acid by a strong base. titration of a weak monoprotic acid. To determine the pka value of a weak acid. In this particular case, the weak base (colored in green), is. Conventional setup of a lab titration. In the previous post, we discussed the. Weak Acid Strong Base Titration Lab Report Discussion.
From snipe.fm
😂 Titration lab report discussion. Acid base TITRATION EXPERIMENT lab Weak Acid Strong Base Titration Lab Report Discussion titration of a weak acid with strong base. titration of a weak acid with a strong base. Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a. To determine the pka value of a weak acid. Conventional setup of a lab titration. titration of a weak acid by a strong base. . Weak Acid Strong Base Titration Lab Report Discussion.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Weak Acid Strong Base Titration Lab Report Discussion titration of a weak monoprotic acid. titration of a weak acid with a strong base. by observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. titration of a weak acid by a strong base. The titration curve shown in. Weak Acid Strong Base Titration Lab Report Discussion.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Weak Acid Strong Base Titration Lab Report Discussion titration of a weak monoprotic acid. Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a. titration of a weak acid with strong base. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. the titration of a weak acid with. Weak Acid Strong Base Titration Lab Report Discussion.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Weak Acid Strong Base Titration Lab Report Discussion The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. titration of a weak monoprotic acid. Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a. In this particular case, the weak base (colored in green), is. titration of a weak acid with a. Weak Acid Strong Base Titration Lab Report Discussion.
From aylinmeowstuart.blogspot.com
Acid Base Titration Experiment Report Weak Acid Strong Base Titration Lab Report Discussion titration of a weak acid with strong base. Conventional setup of a lab titration. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. . Weak Acid Strong Base Titration Lab Report Discussion.
From www.writework.com
Titration of amino acids WriteWork Weak Acid Strong Base Titration Lab Report Discussion To determine the pka value of a weak acid. In this particular case, the weak base (colored in green), is. titration of a weak acid with a strong base. titration of a weak acid by a strong base. by observing the titration of a strong acid and strong base and a strong base and weak acid one. Weak Acid Strong Base Titration Lab Report Discussion.
From mungfali.com
Acid Base Titration Lab Weak Acid Strong Base Titration Lab Report Discussion In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. titration of a weak acid by a strong base. titration of a weak acid with strong base. Figure. Weak Acid Strong Base Titration Lab Report Discussion.
From www.youtube.com
Titration of a weak base with a strong acid Chemistry Khan Academy Weak Acid Strong Base Titration Lab Report Discussion titration of a weak acid by a strong base. Figure \(\pageindex{4}\) shows the four regions of the titration curve for the titration of a. To determine the pka value of a weak acid. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. titration of a weak acid with. Weak Acid Strong Base Titration Lab Report Discussion.